Abstract
The homeostatic immune system in a healthy state represents a dynamic balance between immunogenicity and immune tolerance. Allogenic hematopoietic stem cell transplantation (allo-HSCT) is not only a curative therapy for hematological malignancies, but also has been used to remodel the immune system to a homeostatic state, such as inducing allograft tolerance in solid organ transplantation and treating autoimmune diseases. Therefore, the homeostatic immune system after allo-HSCT represents donor-derived hematopoietic cells that reconstitute the immune system in recipients and establish tolerance to recipients. However, little is known about the responsible genes or relevant cell types that remodel immune homeostasis after allo-HSCT. Moreover, whether haploidentical stem cell transplantation (haplo-SCT) shares a similar pattern of immune homeostasis remodeling with HLA-matched sibling donor transplantation (MSDT) is also unclear.
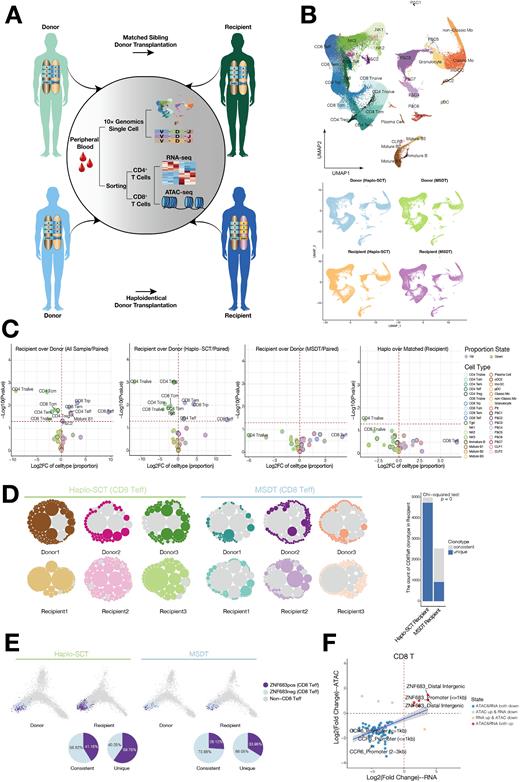
In this study, we used a comprehensive single-cell RNA-seq (scRNA-seq) and single-cell TCR sequencing (scTCR-seq) approach coupled with bulk transcriptome (RNA-seq) and epigenome (ATAC-seq) analysis to dissect the landscape of the rebalanced homeostatic immune system in recipients after allo-HSCT (Fig. 1A). We identified 35 subpopulations in the 5 lineages based on the expression of cell type-associated genes, including the hematopoietic progenitor compartment with highly proliferative ability, T/NK lineage cells, B lineage cells, myeloid lineage cells and platelets (Fig. 1B). All cell subsets were present in both donors and recipients, which indicated that the immune system was successfully reconstituted in the recipients after allo-HSCT (Fig. 1B). Moreover, we found HSPC compartments adequately reconstitute in those recipients who have rebalanced to homeostasis after allo-HSCT. Although all immune cell subpopulations from donors were reconstituted in the paired recipients, there were different patterns of immune subpopulation distributions in recipients after allo-HSCT. In MSDT, donor-derived immune cell reconstitution in recipients as similar to that in their paired donors, while in haplo-SCT, there were low levels of CD4+ and CD8+ naïve T cells, Tcm cells, CD4+ Tem cells and CD4 Treg cells and high levels of CD4+ Teff cells, CD8+ Teff cells, CD8+ Tem cells and CD8+ Trp cells in recipients compared with their paired donors (Fig. 1C). We observed that memory-like CD8 T cells with immunoregulatory functions (CD8 regulatory precursors, CD8 Trp) were expanded in haplo-SCT recipients. CD8 Trp showed similar transcriptional profiling compared with CD4 Treg indicated that increased CD8 Trp subpopulations might act as regulatory cells and contribute to maintaining the tolerance of the donor-derived immune system in recipients of haplo-SCT.
To investigate the dynamic changes in TCR clonotype after allo-HSCT, we integrated the analyzed single-cell transcriptomes and TCR sequences. We found that the dominant clonotype of CD8 Teff cells in MSDT recipients is the clonotype consistent with that in their paired donors, while the dominant clonotype of CD8 Teff cells in haplo-SCT recipients is the recipient's unique clonotype and educated by recipient thymic with high ZNF683 expression (Fig. 1D-E). Combined analysis of bulk CD8 T cell RNA-seq and ATAC-seq, we found that transcription factor ZNF683 was specifically activated in CD8+ T cells from haplo-SCT recipients compared with their paired donors (Fig. 1F). In vitro experiments indicated that a high expression level of ZNF683 in human primary T cells promoted inactivation of CD8+ T cells, maintained CD8+ T-cell replicative capacity and inhibited CD8+ T-cell apoptosis, which might suppress CD8 T cell senescence and corelated with CD8 T cell persistence. Moreover, ZNF683 could be downregulated when CD8+ T cells were activated, which suggests that ZNF683 might be a checkpoint of long-lived quiescent CD8+ T cells.
In summary, our study provides a rich dataset of cellular states, gene programs, and T-cell repertoires in recipients whose donor-derived immune system rebalanced toward homeostasis after allo-HSCT. Understanding the molecular mechanisms underlying different patterns of immune homeostasis remodeling in different transplantation models will help us provide more precise medical care for patients who receive allo-HSCT.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.